On May 27th, Mathieu Horras, Aspivix CEO, will travel to Boston with Venture Leaders Life Sciences and embark on a fully packed one week investors roadshow. Mathieu Horras recently also won the Startup Champions Seed Night pitch competition on May 2nd. Read about his motivation for applying to the program.
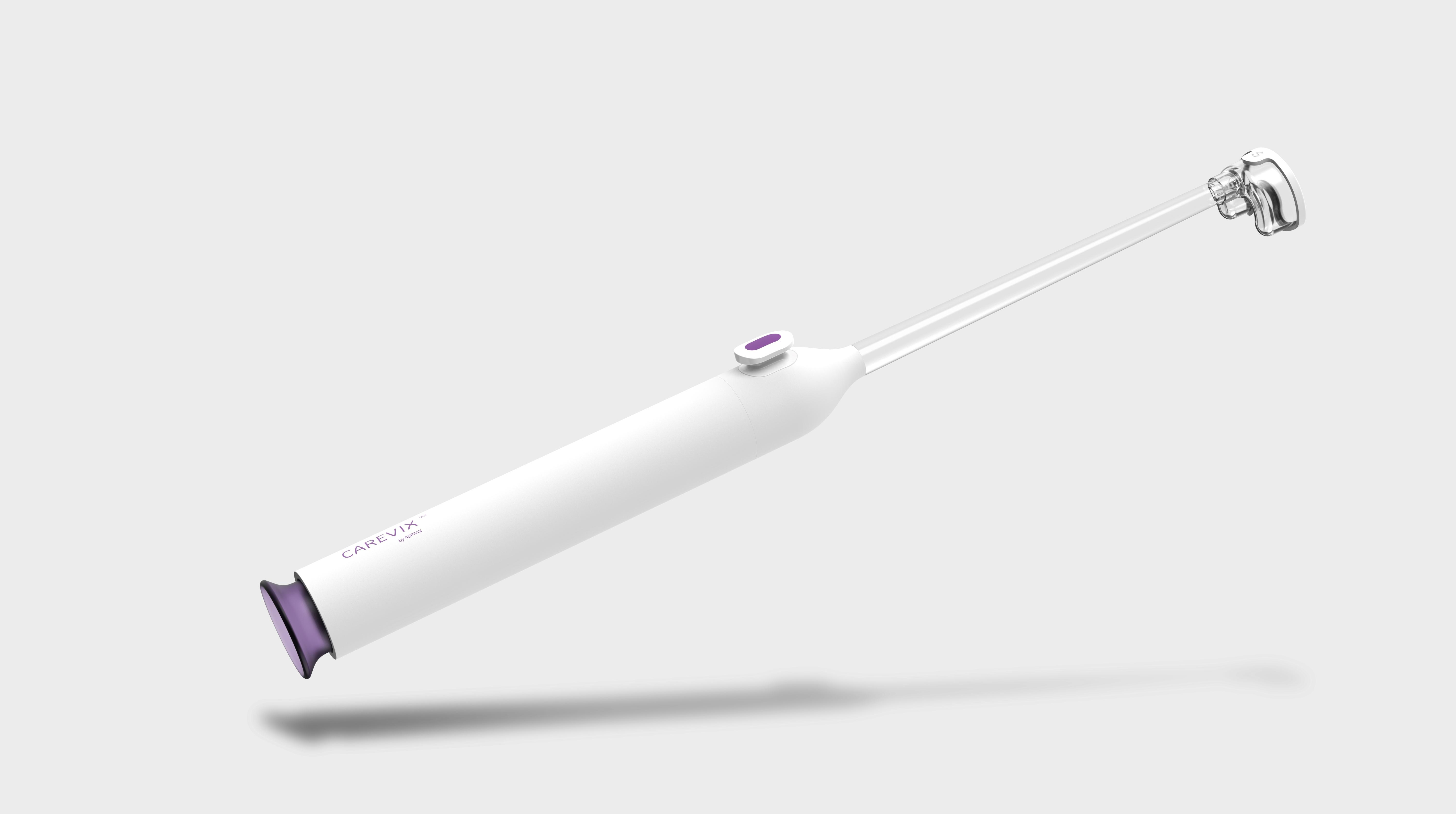
Who are you and what is Aspivix?Every year, 64 mio of gynecological treatments require access to the uterus. Standard use of Tenaculum forceps is painful, traumatic and poses a cross-contamination risk. Aspivix, a new generation of single-use forceps with a gentle and soft vacuum suction pad, reduces pain and eliminates lesions & cross-contamination. By allowing safer access to uterus and improving gynecological procedure such as insertion of an Intra-Uterine Contraceptive Device (IUCD), Aspivix turns out to be the ideal instrument to break IUCD adoption barriers. Aspivix will tackle the pain and contribute to reducing unintended pregnancies.
“The best way to predict the future is to create it.” This inspiring sentence is at the very heart of my motivation to fill a gap in gynecology practice and change the game for millions of women bearing the pain of obsolete practices every year. My vision, shared with my co-founders, is to innovate women’s care and make a daily positive difference. To achieve such a vision, I come with 7-year experience in R&D management, 8-year experience in Global Marketing and Product Development for International Medtech companies as well as my international experience, living in the US, Brazil, Switzerland, France, Singapore, UK, and Japan. Through my education (Engineer by background, I graduated later from an MBA at INSEAD and Wharton), different experiences, in various geographies or industries, I adapted to different cultures to eventually make a difference. Those sources of inspirations taught me to challenge the arguments through various lenses and approaches, constantly searching for innovative yet pragmatic solutions.
Why do you choose Boston as your business development destination?
I see Venture Leaders as a must have and unique opportunity to simultaneously build Aspivix awareness, deal with international investors and turn Aspivix into a global business. Boston is a hotbed for Life Sciences and Health Innovation. Experiencing this program and environment will be mind-opening, will provide numerous great contacts and help to enhance the vision on how to approach the US market and run my company.
What do you hope to achieve from the trip?
Aspivix is currently closing a Series A-round with Swiss VC, Banks and Business Angels. The Series A-round will allow Aspivix to drive all activities required to obtain a CE marking of Aspivix in the 2nd quarter 2019. The next financing round for Aspivix aims at strengthening the claim (less pain, less bleeding) with clinical studies, growing market access and deploying Aspivix beyond Europe and in particular into the US market. In the light of this plan, the expected results from the venture leaders are:
- Sharpen our pitch and go to market strategy (incl. potential approach of payers) for the US by getting feedback from professional US-based VCs
- Network with VCs in order to identify VCs which would be suitable for Aspivix financial second round, adding expertise and funds to win the US market.
- Create a robust, reliable and sustainable network of inspiring other Life Science entrepreneurs to exchange views and perspectives on entrepreneur’s challenges and approaches during the venture leaders and more importantly after.
Venture Leaders will be a unique opportunity to create a ton of key contacts and build a clearer vision on how to drive the US market penetration, all this concentrated in a one-week program.
Why is the Venture Leaders Life Sciences experience beneficial for your company?
Being entrepreneur can be a bit lonely at times. There is often no greater remedy to entrepreneurial loneliness than finding community with fellow entrepreneurs. My ambition is, towards the Venture Leaders team, to create and cultivate a strong and sustainable community of entrepreneurs, who can be reached out during the program and afterward, and who will understand and respect the dedication to the business while understanding the challenges and questions I am facing.
What is pushing you towards international expansion?
Aspivix, which will launch its first product on the market in 2019, is aiming at growing revenues to CHF 27 Mio by 2022 while achieving nearly CHF 10 Mio net profit and expanding to a team of 30 associates. To achieve this performance and get the aimed distribution reach, Aspivix needs to ally with one or more IUCD (Intra-Uterine Device also called spiral) manufacturers. The US market being a key market for IUCD manufactures from a revenue standpoint, it becomes a strategical market for Aspivix in order to gain momentum and embark the IUCD manufacturers into the Aspivix journey.
What do you foresee as the largest hurdles towards expansion into the Boston area?
The US market is a crucial market for Aspivix as US is the largest market in revenues for Intra-Uterine Contraceptive Device (IUCD), the insertion of IUCD being the main indication for the use of Aspivix. Indeed, pain barrier, which is lowered by using Aspivix, is seen by IUCD manufacturers as the primary IUCD adoption hurdle. In the 2nd quarter 2019, Aspivix will initiate a 510(k)-filing process in order to get Aspivix cleared by FDA for the US market. Between 9 and 18 months is nowadays common practice to achieve this key registration step. To penetrate and accelerate the roll-out of Aspivix in the US market, Aspivix will need to identify, evaluate and negotiate with the potential contribution and support from payers and NGOs.
What makes you most nervous or excited about the trip?
50% of the pregnancies in the US are unintended, resulting in between $11bn and $21bn social costs every year in the US. Intra-Uterine Contraceptive Device (IUCD) is the most cost-effective contraceptive solution and is 10 times more reliable than pills. Therefore, gaining wider IUCD adoption is a strong focus for several powerful NGOs in US. NGOs not only encourage the use of IUCD, supporting the costs of it, but they also target younger women (i.e.: teenagers), generally nulliparous patients, who are, according to studies, more subject to pain at insertion of the IUCD. Aspivix, by reducing the pain and the bleeding at the IUCD insertion, will become a key driver of IUCD adoption, hence a social cost reduction driver in the US. This trip will be a door opener to speed up Aspivix’s access to the US market. Thanks to this trip, Aspivix will enable millions of women to choose their contraception without fear, to visit their gynecologist without stress, and to leave without pain. Aspivix will tackle the pain and contribute to reducing unintended pregnancies. This inspiring mission is at the very heart of my motivation.
Follow the Venture Leaders Life Sciences roadshow on Twitter and Facebook using the hashtag #vleadearsLifeScience.
Venture Leaders Life Sciences is co-organized by Venturelab and swissnex Boston, and supported by Kellerhals Carrard, digitalswitzerland, Canton de Vaud, Canton of Zurich, Wyss Foundation, EY entreprenenur of the year, EPF Lausanne, ETH Zurich, Paul Scherrer Institut and University of Zurich.